Remember how health authorities said serious vaccine side effects always will be seen fast?
Turns out that's not true... as Pfizer (and its veterinarian chief executive) should know better than anyone.
In 2010, German scientists found that a Pfizer veterinary vaccine to reduce diarrhea in cows caused a fatal bleeding disease in their calves.
Even after pressure from Germany caused Pfizer to stop selling the vaccine there, the company kept selling it elsewhere. A top Pfizer official told British farmers it was safe to use and that “other factors” were likely involved.
A month later, Pfizer stopped selling the vaccine. European regulators later found it caused a 1-in-6000 risk of the bleeding disease. “For a prophylactic measure such as vaccination this figure was considered unacceptable for a potentially fatal disorder,” the regulators found.
The risk of Covid-vaccine induced myocarditis - which can be fatal - in young men is now estimated at somewhere between 1 in 2000 and 1 in 3000.
Apparently the rules are stricter for cows.
SOURCE: https://www.ema.europa.eu/en/documents/referral/pregsure-bvd-article-78-referral-annexes-i-ii_en.pdf
—
Six weeks after the final dose is plenty of time to discover serious vaccine side effects. Two months is more than adequate. Three is unnecessary.
Both before and after the mRNA Covid vaccines were authorized in 2020, regulators, politicians, and the media insisted on this point. Long-term safety data were not necessary for authorization - because vaccine side effects happen and are caught fast.
According to the Centers for Disease Control: Side effects generally happen within six weeks of receiving a vaccine dose…
SOURCE: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html
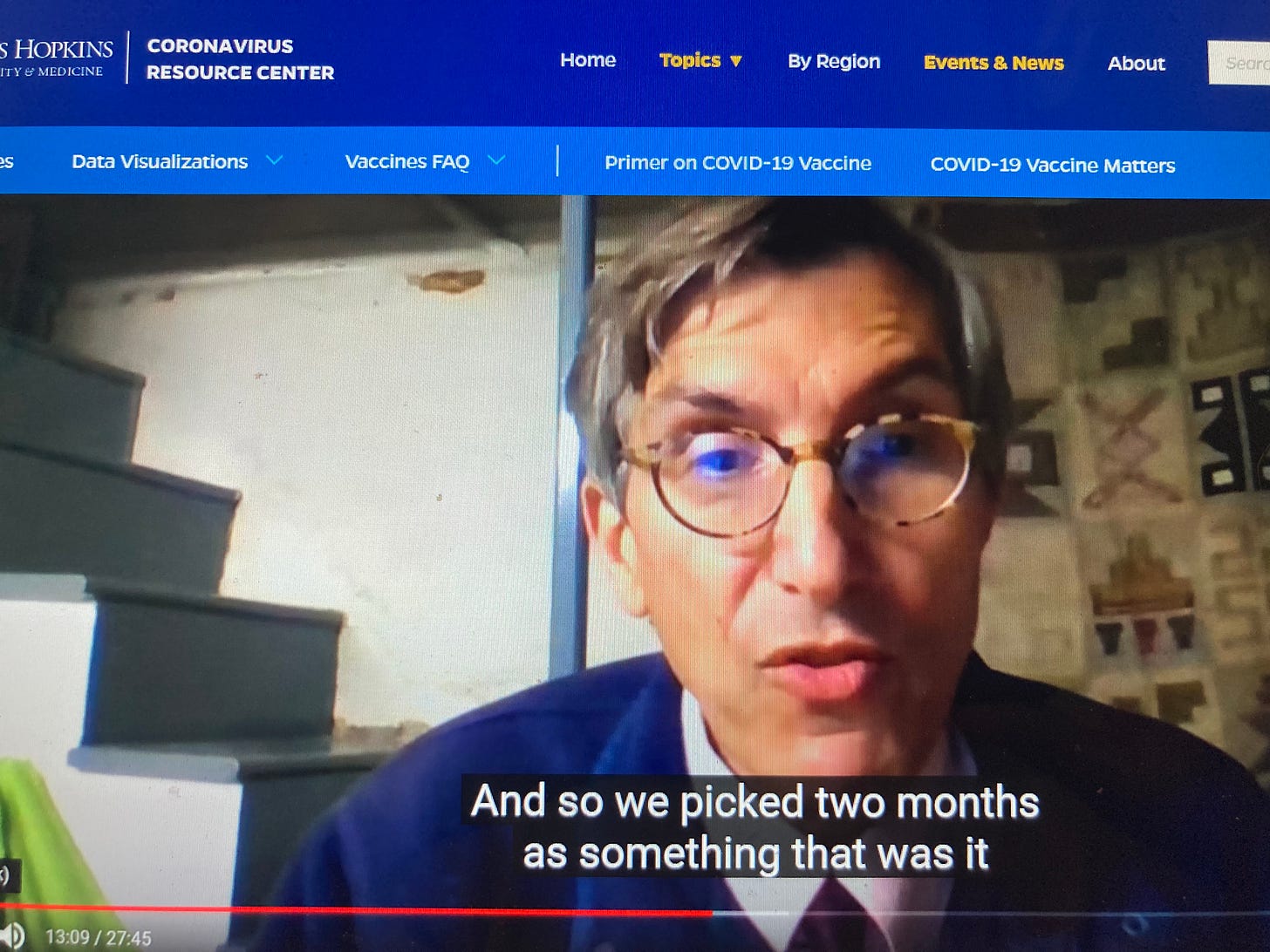
Thus the Food and Drug Administration was actually being conservative when it told Pfizer and Moderna to follow at least half the people who had received the second mRNA dose in their clinical trials for 60 days, vaccine advocates said. At an online conference in October 2020, Dr. Peter Marks, the agency’s top vaccine regulator, said he believed two months was a reasonable standard given the severity of the pandemic and the rarity of long-term vaccine side effects.
That standard meant that fewer than 20,000 people who received mRNA vaccines would be tracked for two months or more - for vaccines that have now been given more than one billion people worldwide.
Dr. Marks of the FDA (from his basement), explaining his views on the amount of time required to be sure a vaccine is safe after its dosing is completed:
—
In 2006, farmers in Germany began seeing calves die horribly.
Between 10 days and three weeks after their birth, the calves would begin bleeding uncontrollably from their eyes, ears, and even their skin. Farmers called the illness “bleeding calf syndrome” or even “blood sweating.”
The disease spread from Germany to other European countries, killing up to 15 percent of calves at some farms. Thousands died. Scientists found that the bone marrow and blood cells of the calves had been destroyed.
They called the disease “bovine neonatal pancytopenia.”
As the outbreaks worsened, scientists, veterinarians, and farmers raced to unearth the underlying cause of the illness. Could the problem be breed-specific? Perhaps it had something to do with the feed the cows received? Or maybe an as-yet-unknown bacterial or viral infection?
The answer was none of those.
Meticulous examination of the records of the calves and their mothers, or dams, revealed that dams who had received multiple doses of a vaccine called PregSure BVD were far more likely to have calves who suffered the “blood sweats.”
Calves who received colostrum - the “pre-milk” fluid that cows and other mammals make before producing breast milk - from vaccinated dams also had a higher risk.
The risk increased with the number of doses that the dams had received, researchers found. Cows that had received at least three doses had 30 times the odds of having a calf with the illness, and those had received at least five doses had a risk more than 40 times as high. They published their findings in a 2013 paper called “Calf-Level Factors Associated with Bovine Neonatal Pancytopenia - A Multi-Country Case Control Study.”
The risk also increased steadily with time - it was far higher in calves whose dams had received the vaccine years insread of months earlier.
SOURCE: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3846664/
Who made PregSure BVD?
Pfizer, through its Pfizer Animal Health group, which Pfizer eventually remained Zoetis and spun out as a separate company. Pfizer introduced PregSure BVD in 2004 and sold more than 14 million doses in Europe alone by 2010. An undated price list shows that the vaccine had a list price of about $10 a dose.
PregSure BVD was designed to prevent the transmission of bovine viral diarrhea from dams to calves; it was an “inactivated” vaccine. That term means the vaccine contained the actual virus, which had been damaged to prevent it from replicating.
Scientists would discover that the vaccine caused some cows to produce “alloantibodies.” The immune system make antibodies to attack foreign invaders in the body, but alloantibodies attack our own red blood cells instead.
These alloantibodies did not attack red blood cells in the dams - the mother cows - but when the calves received them through colostrum they caused terrible damage. Many cows produced the antibodies, but some seemed to make exceptionally high levels of them, and it was calves exposed to colostrum from those cows that became ill.
The connection between the vaccine and the bleeding disorder was evident as early as March 2009, German public health regulators reported in 2011. But Pfizer continued to sell the vaccine in Germany for another year, stopping only weeks before the German government officially opened an investigation into the medicine in late April 2010.
SOURCE: https://www.ema.europa.eu/en/medicines/veterinary/referrals/pregsure-bvd
Even so, Pfizer continued to sell PregSure BVD elsewhere in Europe, and in May, Dr. Ed Ferguson, a company official, told a newspaper for British farmers that “a causal relationship between Pregsure BVD and BNP (bovine neonatal pancytopenia) has not been established.”
A month later, Pfizer stopped selling PregSure BVD worldwide. Even so, the company continued to insist it did not think its vaccine was unsafe.
“"The evidence currently available at European level indicates that BNP is likely to have a multifactorial cause," Pfizer told an Irish newspaper in September 2010, when European regulators officially recalled the vaccine. "The association of BNP with the use of PregSure BVD is not clear.”
Eventually it would be. In the meantime, regulators and Pfizer didn’t continue to expose vulnerable calves to the vaccine.








I'll be on The Ingraham Angle tonight at 10:40 talking about college vaccine mandates how a coalition of parents around the country are fighting against them. Wish me luck!
Alex! Just quickly, I have a family member who has suffered heart inflammation recently after his second jab, and just today, a client told me he was diagnosed with myocarditis last week following his booster. My Mum also has a cousin who has suffered myocarditis following her second shot. So when I read that the risk is estimated at somewhere between 1 in 2000 and 1 in 3000, I just can't see how that is possible. I don't know that many people and yet I know three people (potentially more, who haven't said anything), who have had this issue. It seems much, much more common than between 1 in 2000 and 1 in 3000. Thank you for your excellent work!